Photoreceptors - rods and cones - are highly specialized cells found in the retina; they are able to perceive the light entering the eyes and respond to the light stimulus by generating a nerve impulse that is transmitted to the nerve cells of the internal retina, to the optic nerve and finally to the cerebral cortex, allowing us to see the world around us as well as we know him.
The media have recently given great prominence to the application of gene therapy for the treatment of some ocular pathologies that have been incurable up to now, but let's see what exactly gene therapy consists of, what impact this therapeutic approach has today in the field of ophthalmology and which one it may have in the near future.
Our genetic heritage contains all the "information" necessary to make our body function properly and this information is "contained" in the DNA (deoxyribonucleic acid) in the form of a "code", given by the succession of nucleotides, the subunits that make up DNA . The genetic information is read by the biosynthetic machinery of the cells which thus produces the proteins necessary for the various biological functions of the organism. The stretch of DNA that contains the genetic information that codes for a protein, and therefore for a biological function, is called a gene. Any alteration that causes the loss or damage of a gene, and therefore of its protein / biological function, can lead to the onset of a disease.
Gene therapy aims to introduce a "therapeutic gene" into a patient's cells, that is, an exogenous DNA sequence capable of compensating or replacing a deficient or absent biological function. Therapeutic genes are inserted into the patient's target cells with the aid of viral vectors, ie engineered viral particles, capable of entering specifically into the patient's cells without being pathogenic or immunogenic.
Gene therapy aims to re-establish a deficient or absent biological function due to inherited or acquired genetic defects, in some cases, however, gene therapy can aim to create a new function, such as the ability to produce pharmacologically active molecules to avoid the administration continues, especially where this is particularly uncomfortable or traumatic for the patient.
Gene therapy applied to ocular pathologies is very promising since the eye is an “isolated” compartment from the rest of the organism and therefore the introduction of a viral vector involves a rather low risk of systemic immune reactions.
The administration of the viral vector containing the therapeutic gene can be carried out with different methods:
Intravitreal injection
Subretinal injection
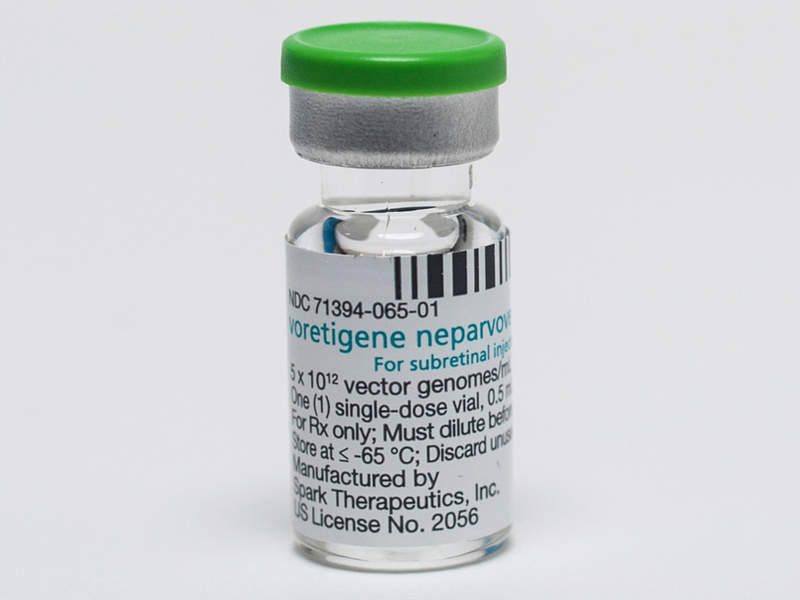
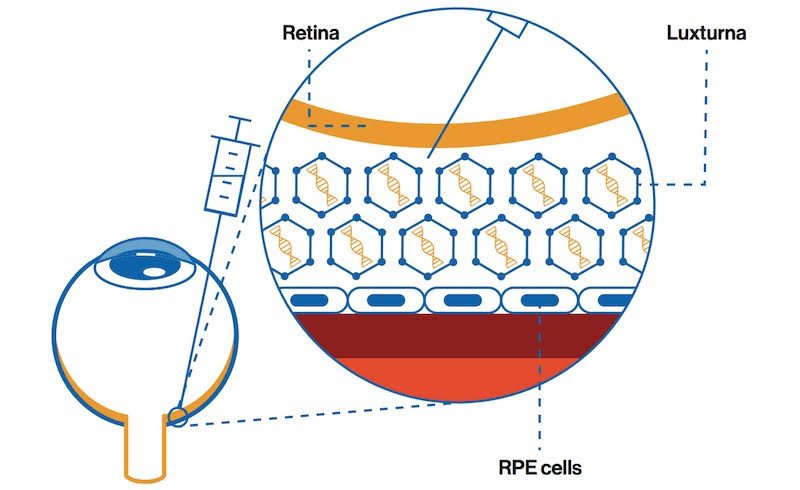
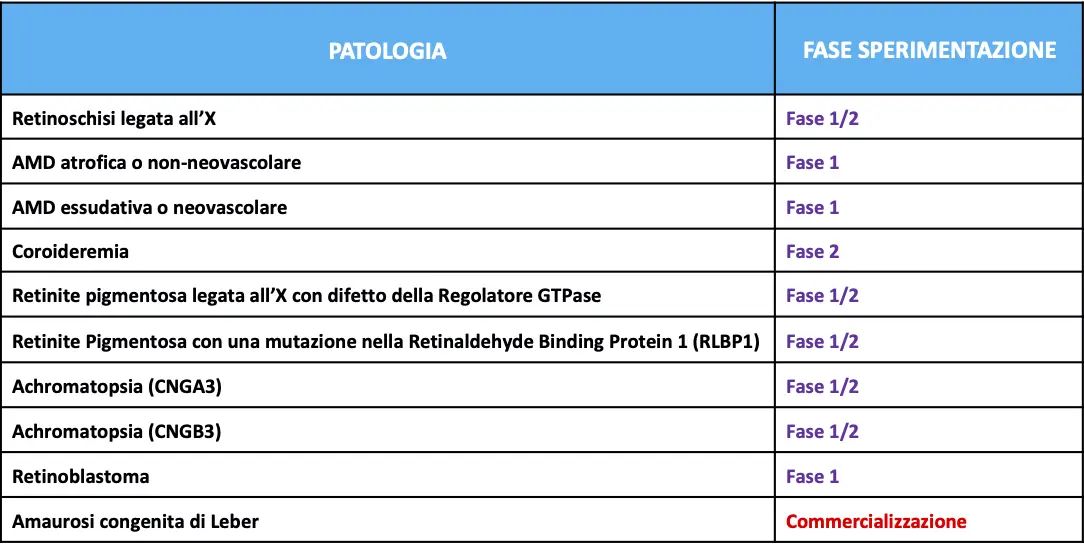
In fact, last December, in the United States the FDA approved the treatment of children and adults suffering from Leber's congenital amaurosis with the new drug LUXTURNA (voretigene neparvovec-rzyl), this event represents a real milestone in the history of the therapy of hereditary retinal diseases and more, since LUXTURNA is also the first example of ocular gene therapy ever
Patients who can undergo this therapy are those who have a biallelic mutation of the RPE65 gene associated with retinal dystrophy and characterized by a lowering of vision that in some patients leads to blindness. The RPE65 gene encodes a 65kDa protein synthesized in the cells of the retinal pigment epithelium and essential for the conversion of trans-retinol to 11-cis-retinol and subsequently to 11-cis-retinal, a key reaction of the visual cycle. In these patients, the mutation of the RPE65 gene results in a reduction or total absence of the activity of the protein, with consequent blockage of the visual cycle and reduction of vision. LUXTURNA aims to insert a healthy RPE65 gene into retinal cells to restore the production of a normal protein capable of converting light into an electrical signal, thus restoring the visual cycle. LUXTURNA is administered by a subretinal injection of 0.3 mL of drug and the therapeutic gene is carried by a modified adenovirus using DNA recombinant techniques. Treatment should be carried out in the two eyes separately and the patient should take oral prednisone doses before and after each injection in order to avoid a potential immune reaction to the drug.
Sparks Therapeutics, which developed the drug, is working with doctors and health insurers to ensure patients have access to this product. We can finally say that today we have the first gene therapy for a hereditary-familial retinal disease and we hope that this milestone is the precursor of many other innovative therapies that will be able to counteract this category of pathologies that today cause very serious visual disabilities, weighing heavily on the life of a great multitude of patients.